Overview
An American multinational pharmaceutical and biotechnology company founded in Chicago in the 1800’s. The company’s mission is to improve global healthcare outcomes by developing innovative and accessible pharmaceutical solutions.
Business Objectives
In today’s hyper-competitive world of pharmaceuticals, the race to bring a new drug to market is fierce. Pharma companies are no longer just competing with rivals, but also with macro challenges, including increasingly stringent regulations, escalating R&D costs with changing patient demographics and disease patterns.
To succeed, pharma companies must continuously innovate, introducing products that are not just superior, but also more cost-effective than those of their competitors.
The client recognized the strategic imperative of understanding their changing competitive landscape to enhance its product strategy. The client was interested in not just tracking the achievements and successes of their competitors, but more importantly, their missteps and failures.
They understood that competitors’ negative developments can offer vital insights that could sharpen their product strategies and innovation efforts. In addition to the overall monitoring of the competitive landscape, the client was specifically interested in monitoring the following negative developments:
Product Recalls
Monitoring product recalls uncovers potential weak points in competitors’ offerings, provides insights into safety issues, and reveals possible regulatory or compliance concerns flagged by the FDA. By doing so, the client would be better positioned to learn from their competitors’ mistakes and proactively adjust their product positioning.
Halted Clinical Trials
Tracking competitors’ clinical trials – especially those that are prematurely halted – could provide a wealth of insights to the client. Understanding the reasons behind a trial’s termination sheds light on the efficacy and safety of developing drugs, empowering companies to make informed decisions about their own product pipeline, competitive positioning, and potential collaboration or licensing opportunities.
Patent Infringements
By keeping an eye on competitors’ patent infringement cases, the client could gain valuable insights into potential threats to intellectual property rights and detect competitor activities that could lead to legal disputes. Moreover, tracking patents can help companies stay informed about emerging trends and technologies in the competitive landscape, ultimately safeguarding their innovations.
Delayed Product Launches
Monitoring product launch timelines, including any delays, is a critical business strategy. Awareness of these delays could allow the client to seize market opportunities and adjust their marketing and sales strategies. Additionally, understanding the reasons behind these delays could provide insights into potential hurdles in drug development and regulatory processes, enabling the client to navigate similar challenges more effectively.
Rejections by CHMP
Tracking instances where competitors’ applications are not recommended or approved by the Committee for Medicinal Products for Human Use (CHMP) could offer invaluable regulatory insights. The client would be able to identify potential improvement areas in their product development based on understanding the reasons behind such rejections, enhancing their ability to successfully navigate the regulatory approval process.
Facility Closures
Staying informed about competitor facility closures is essential for strategic planning. Awareness of such disruptions could allow the firm to identify potential supply chain issues, assess market impact, and strategize to fill any market gaps left by these closures.
Key Challenges
Navigating Challenges in Tracking Negative Competitor Events
While understanding the objectives and benefits of monitoring competitors’ negative events, the client confronted a multitude of execution hurdles. In this section, we delve into the specifics of these challenges.
Inconsistencies in Manual Tracking
A significant issue was inconsistencies in manually tracking of negative developments. With the sheer volume of data across different sources, it was challenging to manually review, capture and monitor relevant information. This labor-intensive process was not only time-consuming but also led to missed critical updates, catching the client off-guard. The client needed a solution to automate this process, ensuring real-time and comprehensive coverage of important updates.
Keywords (not concepts) Dependent Tracking
Finding the right keywords for effective tracking was another challenge for the client. Amidst a deluge of competitor information, identifying the most pertinent keywords to capture adverse events and triggers required considerable precision. Any mistake in choosing keywords could create blind spots in the monitoring process. The client sought a system capable of finding information based on concepts, not just keywords. Not all mentions related to product recalls had “product recalls” keyword in them. Similarly, patent infringement, facility closures, and other key events, need to be tracked irrespective of the keywords. Such competitive intelligence requirements need a platform that heavily leverages artificial intelligence.
Disseminating Negative News to Internal Stakeholders
Once the client identified relevant negative news and adverse events, the challenge was to effectively disseminate this information to internal stakeholders. As stakeholders were not reading all their emails, it was important to integrate the competitive intelligence into their existing workflows. Timely sharing of such crucial updates was vital for prompt decision-making and devising effective response strategies. The client needed a streamlined communication process to deliver these updates to the relevant stakeholders, ensuring they were well-informed and equipped to respond to potential risks or opportunities.
Extended Turnaround Times
Lengthy turnaround times in capturing, analyzing, and communicating key negative events was another hurdle for the client. Traditional methods of tracking and monitoring adverse events often led to delays in gathering insights and initiating necessary actions. The client sought a solution that could provide real-time updates, reduce manual data collection and analysis time, and deliver actionable intelligence promptly. Streamlining the workflow to reduce overall turnaround time was critical to enable quick decision-making and sustain a competitive advantage.
Understanding the need for an advanced market and competitive intelligence platform to address these challenges, the client evaluated multiple vendors. After thorough due diligence, they chose Contify, as its cutting-edge technology and comprehensive features closely aligned with the client’s needs.
Contify Solution
To meet the client’s objectives and address the challenges they encountered in their pursuit, Contify deployed a bespoke platform designed to meet the client’s specific needs. Here are some key components of this solution:
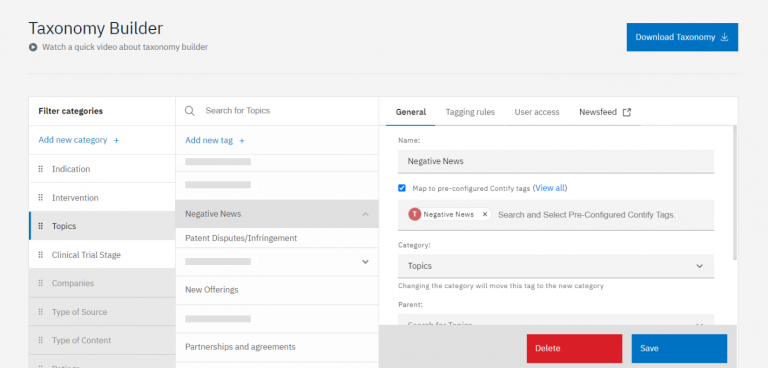
Custom Taxonomy
The foundation of the platform was a custom taxonomy, encompassing tags and keywords to track negative triggers and adverse mentions of competitors, areas of interest, and crucial competitor events and developments.
Custom Sources
Contify integrated several custom sources specific to the client’s requirements into the platform, including:
- The FDA (Food and Drug Administration) Database for regulatory updates, product recalls, and clinical trial information.
- Patent Databases to monitor patent infringement cases and track competitor activities related to intellectual property rights.
- Regulatory Authorities Websites to track application recommendations and approvals by CHMP, staying informed on the regulatory landscape.
- Industry News and Publications to capture competitors’ product launch delays, facility closures, and other key negative events.
In addition, custom rules were added to automatically reject irrelevant updates.
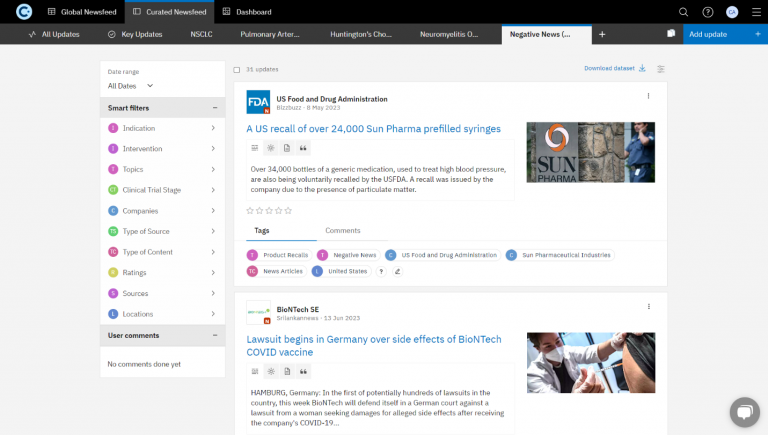
Curated Newsfeed
The newsfeed was configured using the custom taxonomy and sources, equipped with Smart Filters. These filters allowed the client to track competitors’ negative triggers like product recalls, layoffs, facility closures, FDA observations, unsuccessful price negotiations, adverse drug events, halted or paused clinical trials, applications not recommended for approval by CHMP, patent infringements, delayed product launches, and more.
Newsletter and Email Alerts
Contify’s Newsletter and Email Alert features were utilized to optimize the content delivery workflow, ensuring stakeholders stayed abreast with the latest negative competitor developments.
The Newsletter feature sent curated and personalized digests of crucial news and insights directly to the clients’ inbox.
The Email Alerts feature offered real-time notifications about specific negative triggers and events identified by the platform. Clients could customize these alerts to receive instant notifications when new information became available.
Integration with Slack
One of the standout features was the integration with Slack, where the most critical negative events were shared instantly, keeping everyone informed and aligned. Notably, selected comments made on the Slack channel were fed back into the Contify platform. This innovative process was crucial in incorporating this “primary intelligence” into the common repository of negative events, thus creating an effective and collaborative intelligence loop.
Dashboards
Contify’s dashboards empowered the client to visualize and analyze competitors’ negative news and updates. These interactive dashboards, equipped with various widgets, enabled the client to pinpoint negative news by geography, identify competitors’ problem areas, track patent disputes, and analyze product recalls by companies through insightful visualizations.
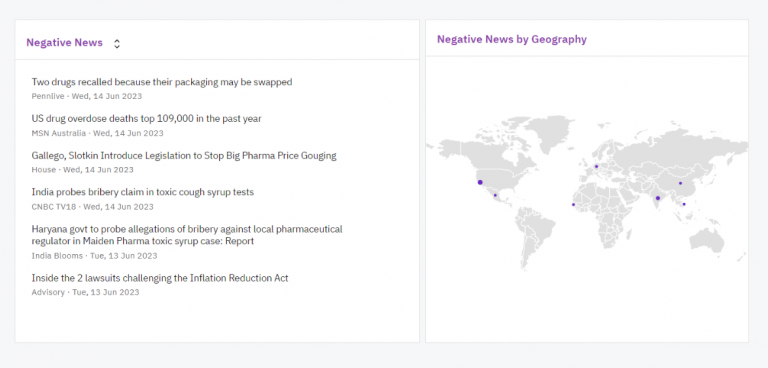
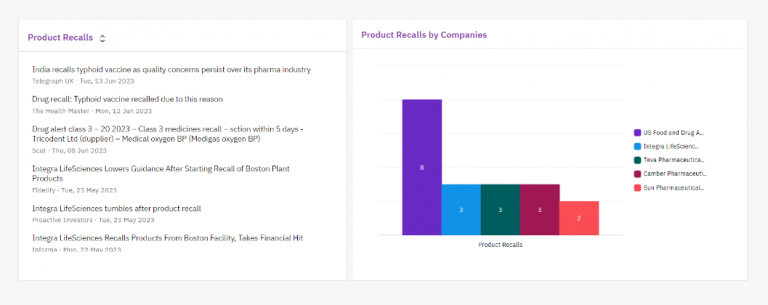
This user-friendly interface facilitated the client in making data-driven decisions.
Impact
Enhanced Competitive Advantage
With Contify’s platform, the client now has access to real-time updates and comprehensive coverage of key competitor events, including product recalls, halted clinical trials, patent infringements, and more. This wide-range visibility allows the client to pinpoint vulnerabilities in competitors’ product lines and make informed decisions about their own drug development pipeline, creating a clear competitive edge.
Quick Decision-Making and Response
The platform also offers the client an efficient way to disseminate crucial negative news and adverse event information to internal stakeholders. The timely distribution of these updates equips stakeholders to make prompt, informed decisions and construct effective response strategies.
Reduction in Turnaround Time (TAT)
Contify’s automated alerts, ML-based news tagging, and customized taxonomy have helped the client to reduce the record-to-report phase’s turnaround time by 2.5 times. This considerable time and resource optimization frees the client to concentrate on strategic initiatives, such as product development, market expansion, and fostering collaborations.
Customer Testimonial
The client affirmed the effectiveness of Contify’s platform, stating:
“Contify’s platform has greatly optimized our time and resources. Their system has not only helped us monitor competitors’ negative news but also uncovered several blind spots in our own strategy. Now, we’re better equipped to enhance our product innovation efforts by capitalizing on competitors’ pain points.”







